Breaking Through the Cell Barrier: Portal's Direct-to-Biology Drug Screening Solution
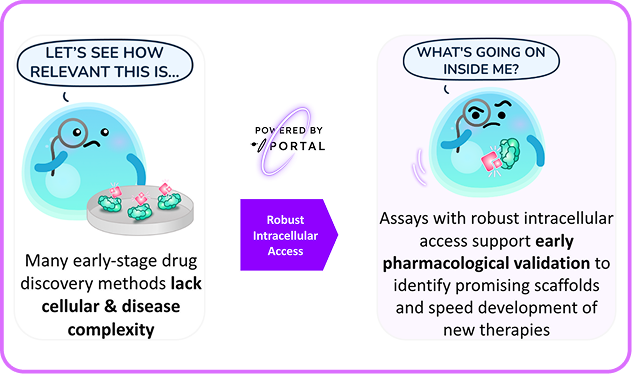
Early-stage drug discovery has a permeability problem. Promising compounds that shine in Biochemical assays often fail when they encounter living cells. This fundamental disconnect between biochemical promise and cellular reality has created a frustrating bottleneck in modern drug development and has in part inspired a movement toward Direct-to-Biology drug screening
The $100 Billion Permeability Problem
Traditional drug discovery follows a predictable path: screen compounds in cell-free systems, identify promising hits, then test them in cells. It's a logical workflow with one critical flaw—many therapeutic targets simply can't be properly studied outside their native cellular environment. Membrane proteins lose their structure, multi-protein complexes fall apart, and essential cofactors disappear.
The result? As one comprehensive study notes, "compounds with excellent biochemical activity may show poor cellular potency" if they can't cross cell membranes1. Teams waste months improving cellular permeability of scaffolds that are ultimately ineffective in the complex cellular environment and may abandon attractive scaffolds that could demonstrate high activity in cells.This permeability bottleneck is particularly devastating for next-generation therapeutics. Peptides, macrocycles, and heterobifunctional compounds are some of the most promising frontiers in drug discovery—often require significant investment before a compound can directly modulate its intracellular target.
The Portal Solution: Squeezing Past Biology's Barriers
Portal's breakthrough lies in mechanoporation—essentially "squeezing" cells to temporarily open their membranes. The technology flows cells through precisely engineered pores, causing rapid deformation that transiently ruptures the plasma membrane. In that brief moment, surrounding molecules rush directly into the cytosol before the membrane self-heals and traps the cargo inside2,3.
Past research has demonstrated successful delivery of everything from small fluorescent tracers to large proteins and nucleic acids into notoriously difficult primary immune cells3. The platform's versatility is striking: it delivers structurally diverse molecules while processing millions of cells per minute, all while maintaining cell viability. Unlike harsh chemical or electrical methods, mechanoporation works on fragile primary cells with minimal functional impact—there is a reported 10-fold better reprogramming efficiency compared to electroporation2.
From Test Tube to Truth: Direct Cellular Screening
Portal fundamentally transforms drug screening by enabling entire libraries to be tested directly inside living cells. No more guessing whether failures result from poor scaffolds or permeability. The technology answers the critical question immediately: does this compound actually work when it reaches its target? Research using mechanoporation showed that poorly permeable kinase inhibitors achieved up to 90% pathway inhibition once delivered directly to primary cells1.
Accelerating Discovery, Reducing Failure
By eliminating permeability as a variable, Portal enables medicinal chemists to focus on scaffolds that have demonstrated phenotypic potential. As one study emphasized, mechanoporation "bridges the gap between biochemical and cellular studies," enabling clearer target engagement assessment and more effective lead optimization1.Portal's mechanoporation platform represents more than a technical advancement—it's a paradigm shift toward authentic biological screening. By enabling researchers to test any molecule directly in its intended cellular environment, Portal helps accelerate drug discovery.The question is no longer whether a compound can cross the cell membrane.
The question is: once it gets inside, does it work? Mechanoporation ensures you'll know the answer.
1 Li, J., Wang, B., Juba, B. M., Vazquez, M., Kortum, S. W., Pierce, B. S., Pacheco, M., Roberts, L., Strohbach, J. W., Jones, L. H., Hett, E., Thorarensen, A., Telliez, J. B., Sharei, A., Bunnage, M., & Gilbert, J. B. (2017). Microfluidic-Enabled Intracellular Delivery of Membrane Impermeable Inhibitors to Study Target Engagement in Human Primary Cells. ACS chemical biology, 12(12), 2970–2974. https://doi.org/10.1021/acschembio.7b00683
2 Sharei, A., Zoldan, J., Adamo, A., Sim, W. Y., Cho, N., Jackson, E., Mao, S., Schneider, S., Han, M. J., Lytton-Jean, A., Basto, P. A., Jhunjhunwala, S., Lee, J., Heller, D. A., Kang, J. W., Hartoularos, G. C., Kim, K. S., Anderson, D. G., Langer, R., & Jensen, K. F. (2013). A vector-free microfluidic platform for intracellular delivery. Proceedings of the National Academy of Sciences of the United States of America, 110(6), 2082–2087. https://doi.org/10.1073/pnas.1218705110
3 Sharei, A., Trifonova, R., Jhunjhunwala, S., Hartoularos, G. C., Eyerman, A. T., Lytton-Jean, A., Angin, M., Sharma, S., Poceviciute, R., Mao, S., Heimann, M., Liu, S., Talkar, T., Khan, O. F., Addo, M., von Andrian, U. H., Anderson, D. G., Langer, R., Lieberman, J., & Jensen, K. F. (2015). Ex vivo cytosolic delivery of functional macromolecules to immune cells. PloS one, 10(4), e0118803. https://doi.org/10.1371/journal.pone.0118803